ISO 13485 Certification In Philippines
ISO 13485 Certification in the Philippines
ISO 13485 Certification In Philippines, Certivatic is specialized in providing ISO 13485 Certification and Consultation in Philippines. We provide the best ISO Consultant services in Cebu City, Manila, Pasig, Makati, and other major cities in Philippines with the service of consultation, implementation, documentation, training, auditing, and registration. We do provide CE mark Certification, HALAL Certification around the world at an affordable cost.
ISO 13485 Certification in Philippines & its significance
ISO 13485 Certification in Philippines is one of the internationally recognized standards which specify the requirements for quality management system for the organizations which are involved in the manufacturing of medical devices at all the stage of products life cycle. The process includes development, production, design, distribution, storage, installation, services and other technical supports for the device. It is also applicable for other parties who provide materials, services or products to the organization and can be applied to any size.
The standard focuses on the process based approach related to quality management within industries. And it is a review of the series, the outputs and inputs and other interactions of the process. The process based approach enables the management system not as a collection of documents and records but as an active system of the processes and procedures. ISO 13485 Certification in Philippines helps the organization to identify and mitigate the risk and threats involved in the production activities so that the end products are away from the uncertainties.

Get Free Consultation
Downloads
ISO Certification In Philippines
- ISO 9001 Certification In Philippines
- ISO 14001 Certification In Philippines
- ISO 22000 Certification In Philippines
- ISO 27001 Certification in Philippines
- ISO 45001 Certification in Philippines
- ISO 13485 Certification in Philippines
- ISO 17025 Certification in Philippines
- CE Mark Certification in Philippines
- Halal Certification in Philippines
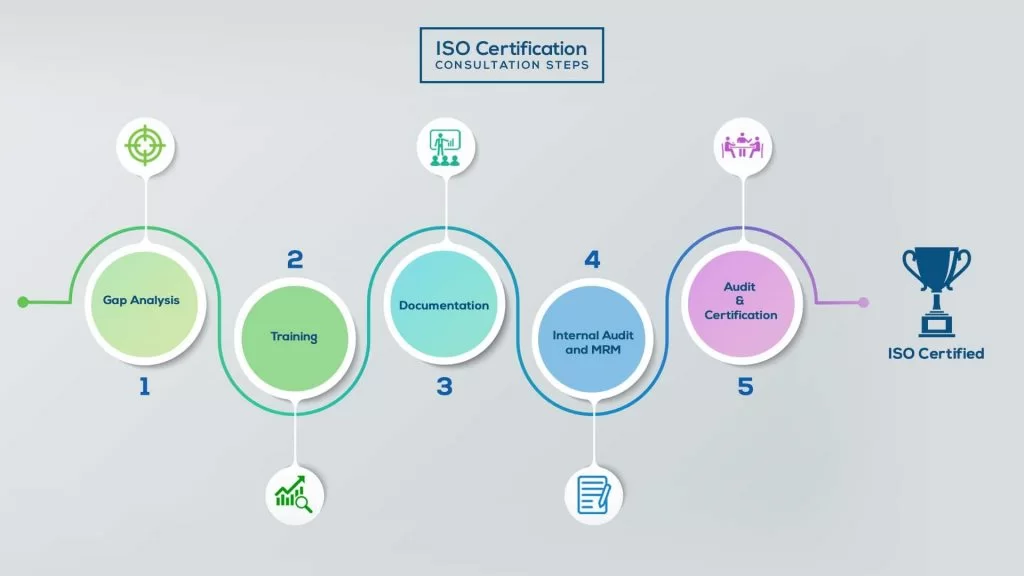
Our method/steps are easy, unique, time-bound, and result-oriented.
Gap Analysis:
- Interacting and detailed study of current work flows in different departments, operating procedures, documentation and practices.
- Identifying any shortcomings of your organization against the requirements of ISO.
- Generating a Gap report and planning for implementation
Training:
- Creating awareness about ISO standard and its importance.
- A formal training session/s.
- It includes training on internal auditing, documentation training, mock audit and trainings to conduct management review meeting.
Documentation:
- Documentation is the manifestation for your entire organizations process, procedure and results.
- End-to-End support on preparing documentation as per ISO requirements.
Internal Audit & MRM:
- Internal Audit (IA) as a tool, it is a cross departmental audit to ensure zero loophole in the system of your organization.
- MRM: Management review meeting (MRM) helps top management to guide and ensure the whole organization is up to the mark as per the standard requirements.
Final Audit and Certification:
- Certivatic – Your certification is our responsibility!
- With our 100% track record of success, we make sure Certification is achieved successfully.
Importance of ISO 13485 Certification in Philippines
The main aim of this international standard is to maintain the highest quality in the field of distribution, manufacturer, maintenance and use of medical devices. If the end products are of not good quality then there will be a major issue in industries that would result in substantial financial impacts and there will be loss of brand Integrity. Hence the organization has to opt for ISO 13485 standards in order to overcome these kinds of issues and let us see some more potential benefits
- It helps the organization by providing and improved quality products that will result in the improvement of brand Integrity.
- It helps to enhance the customer satisfaction by providing high quality products which will lead to repeated businesses
- Improvement in the efficiency and reducing the costs
- It helps in aligning with the strategic goals by involving decision based approaches
- It is one of the important tool in achieving the continuous improvement and customer satisfaction.
- It helps the organization to comply with all kind of legal requirements whether it may be statutory or regulatory along with the client’s requirements.
Hence ISO 13485 Certification in Philippines plays a vital role in achieving the organizational goals by providing the requirements which is been accepted globally.
For More Information visit: ISO 13485 Certification in Philippines


