ISO 13485 Certification In India
ISO 13485 Certification in India
As one of the leading ISO Certification providers in India, Certivatic offers ISO 13485 Certification. Our ISO Consultant services are available in Delhi, Mumbai, Bangalore, Chennai, Hyderabad, and other major cities.
An ISO 13485 certification in India is an international standard based on the requirements of quality management systems for medical devices. As of 1996, the International Organization for Standardization provided the requirements for medical device compliance. This particular international standard in Mumbai is ISO 13485, which is based on the quality management system in the design and manufacturing of medical devices.
What is ISO 13485 certification in India and the purpose of implementation?
ISO 13485 certification in India focus on medical device industries where the requirements of quality management system is necessary with respect to medical industries. ISO 13485 standard is one of the well-known International standards published by the international organisation for standardization widely used for quality management system. ISO 13485 certification in India support the management system in order to establish the problems addressed by the medical device derivatives in the medical industry. Implementation of ISO 13485 standard also ensure the safety and the quality of medical devices that exist in the organisation. Similarly like to the widely quality management system standard, the identification of customer requirements is one of the benefits that comes along with implementation of ISO 13485 standard. Along with fulfilling customer satisfaction providing the documentation regarding medical devices quality policies and setting up objectives, the role of ISO 13485 standard comes into picture. Many organisations in medical industries go with the implementation of ISO 13485 certification in India in order to obtain a positive result and recognition such as International organisation across the world.

Get Free Consultation
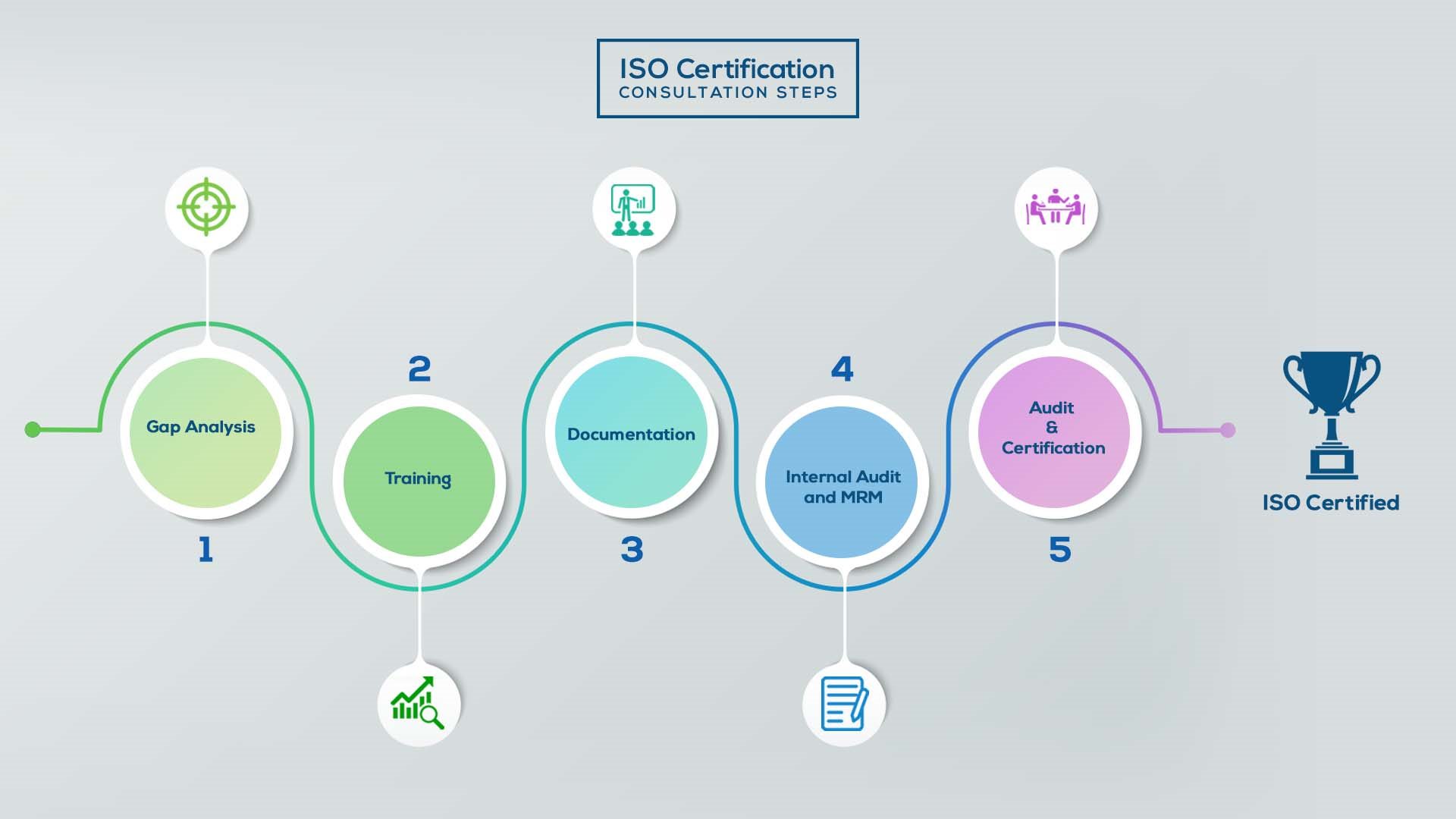
ISO Certification In India
Our method/steps are easy, unique, time-bound, and result-oriented.
Gap Analysis:
- Interacting and detailed study of current work flows in different departments, operating procedures, documentation and practices.
- Identifying any shortcomings of your organization against the requirements of ISO.
- Generating a Gap report and planning for implementation
Training:
- Creating awareness about ISO standard and its importance.
- A formal training session/s.
- It includes training on internal auditing, documentation training, mock audit and trainings to conduct management review meeting.
Documentation:
- Documentation is the manifestation for your entire organizations process, procedure and results.
- End-to-End support on preparing documentation as per ISO requirements.
Internal Audit & MRM:
- Internal Audit (IA) as a tool, it is a cross departmental audit to ensure zero loophole in the system of your organization.
- MRM: Management review meeting (MRM) helps top management to guide and ensure the whole organization is up to the mark as per the standard requirements.
Final Audit and Certification:
- Certivatic – Your certification is our responsibility!
- With our 100% track record of success, we make sure Certification is achieved successfully.


What are the key benefits of ISO 13485 certification in India?
Process improvement: with the help of implementation of ISO 13485 standard the organisation can eliminate wastage. This particular action will help the organisation in reducing the error and removes unnecessary wastage of resources. This will improve the overall efficiency of the team which also act as a cost saving method.
Documentation methodologies: ISO 13485 standard requires certain set of rules and responsibilities that must be briefed in order to establish medical device files in a systematic way. this will also boost up the working environment of each individual in the medical field.
Control establishment: management system must have controls over each and every department in order to perform accurately according to the framework. This will help the organisation in achieving the goals and aligning the prospects of the company intact.
Customer satisfaction: by setting up a quality management principle with respect to ISO 13485 standard the organisation can make sure customer satisfaction is achieved at all point of time. some organisation goes with the implementation of ISO 13485 standard in order to fulfil the customer requirements.
ISO 13485 certification in India assumes an extremely significant function in having the quality administration framework in a legitimate method of actualized in the association where they are including with the assembling and planning of clinical gadgets.


