ISO 13485 Certification in Lebanon
ISO 13485 Certification in Lebanon
ISO 13485 Certification In Lebanon, Certivatic is specialized in providing ISO Certification and Consultation in Lebanon. We provide the best ISO Consultant service in Beirut, Tripoli, Sidon, Byblos, Baalbek, and other major cities with the service of consultation, implementation, documentation, training, auditing, and registration. We do provide CE mark Certification, HALAL Certification around the world at an affordable cost.
ISO 13485 certification in Lebanon & its significance
ISO 13485 Certification in Lebanon is one of the important quality management systems for medical devices which make sure that all the medical devices meet the regulatory compliance laws and customer need &expectations. The requirements of ISO 13485 standard provides a place to keep the experts and other customers safe in hospitals, clinics and other medical setups.
ISO 9001 is a basic standard where all the other standards follows this model approach and ISO 13485 follows this model approach which is specifically developed for the manufacturing of devices related to medical industry. The main aim or goal of an ISO 13485 standard is to provide harmonized medical device regulatory requirements.
The objective of ISO 13485 certification in Lebanon is to reduce legal and safety risk while creating more economical working conditions. It is one of the internationally recognized standards specifying the requirements for quality system and safety system for medical device manufacturing industries. It helps the medical industries to get more recognition that it is more trustworthy and reputable providers.
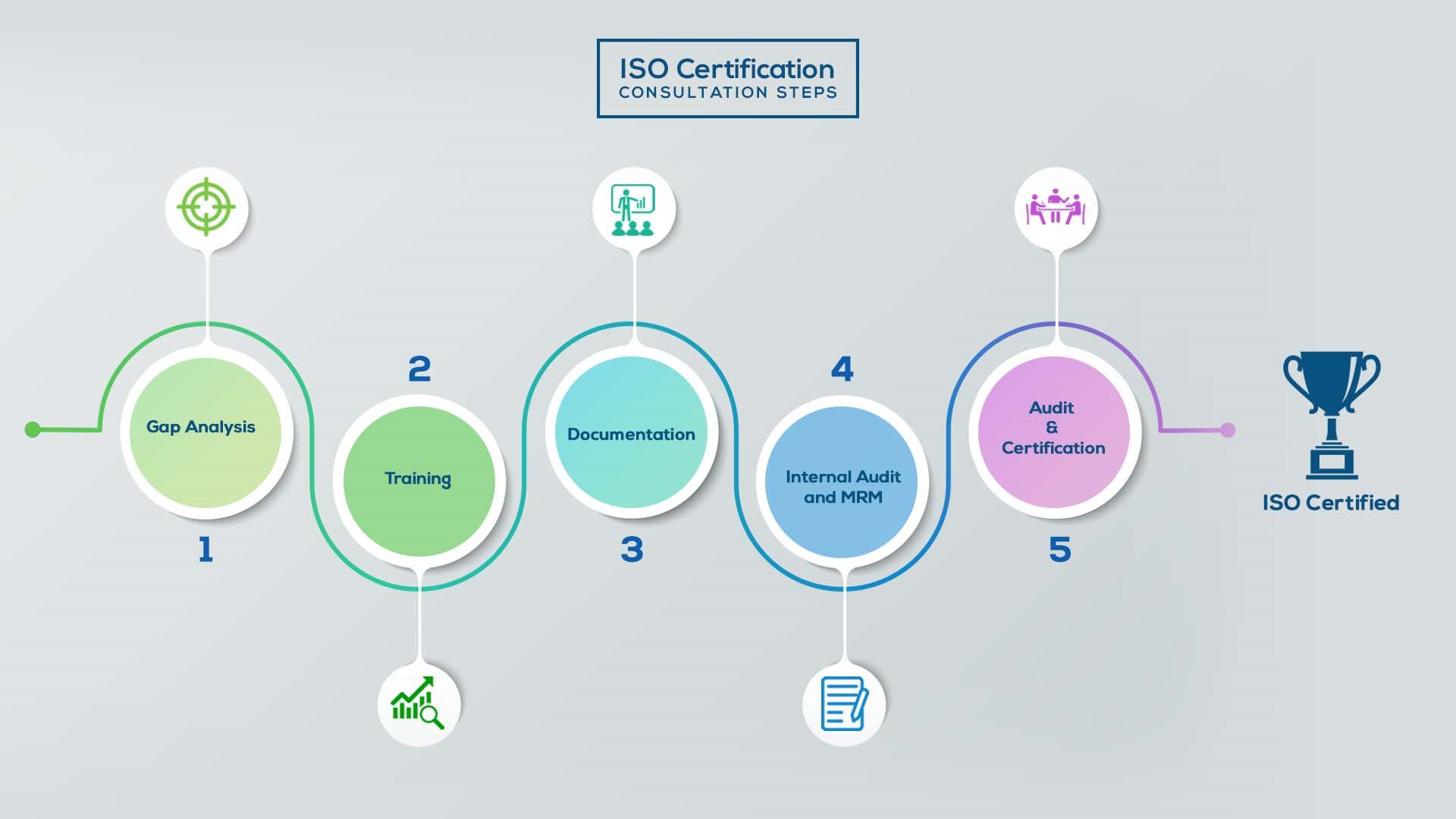
How to get ISO 13485 certification in Lebanon? – Our effective implementation steps:

Get Free Consultation
ISO Certification In Lebanon
- ISO 9001 Certification In Lebanon
- ISO 14001 Certification In Lebanon
- ISO 22000 Certification In Lebanon
- ISO 27001 Certification in Lebanon
- ISO 45001 Certification in Lebanon
- ISO 13485 Certification in Lebanon
- ISO 17025 Certification in Lebanon
- CE Mark Certification in Lebanon
- Halal Certification in Lebanon
Our method/steps are easy, unique, time-bound, and result-oriented.
Gap Analysis:
- Interacting and detailed study of current work flows in different departments, operating procedures, documentation and practices.
- Identifying any shortcomings of your organization against the requirements of ISO.
- Generating a Gap report and planning for implementation
Training:
- Creating awareness about ISO standard and its importance.
- A formal training session/s.
- It includes training on internal auditing, documentation training, mock audit and trainings to conduct management review meeting.
Documentation:
- Documentation is the manifestation for your entire organizations process, procedure and results.
- End-to-End support on preparing documentation as per ISO requirements.
Internal Audit & MRM:
- Internal Audit (IA) as a tool, it is a cross departmental audit to ensure zero loophole in the system of your organization.
- MRM: Management review meeting (MRM) helps top management to guide and ensure the whole organization is up to the mark as per the standard requirements.
Final Audit and Certification:
- Certivatic – Your certification is our responsibility!
- With our 100% track record of success, we make sure Certification is achieved successfully.
Requirements of ISO 13485 certification in Lebanon during installation, manufacturing and service
- Implementation of quality management system requirements with several improvements
- For product realization and product development risk management approach is applied
- Process validation
- Meeting the requirements related to statutory and regulatory
- Product traceability and recall systems should be more effective
It helps the organization to demonstrate consistent development, production, design, storage installation, distribution, servicing, decommissioning and disposal of devices and other activities related to design and development. The requirements of the quality management system for medical device involves in entire life cycle of manufacturing process of medical devices in the industries and it can be applied to the organization irrespective of size and type.
ISO 13485 can be used by the suppliers or by the external parties such as a distributor, manufacturers and so on for providing the services or products to medical device industries. Implementing the requirements of ISO 13485 certification in Lebanon by external party or supplier might be an independent choice or it can be mandatory for certain legal requirements given by the country or by contractual request from their clients.


